Paxlovid Guidelines 2025. As of october 13, 2025, hhs and pfizer reached an agreement extending patient access to paxlovid, maximizing taxpayer investment, and beginning paxlovid’s transition to the. While the stanford trial did not show that.
While the stanford trial did not show that. [01/29/2025] in december 2025, fda authorized paxlovid for emergency use for the treatment of adults and pediatric patients (12 years of age and older weighing at least 40.
The documented share of covid tests in california that came back positive has risen from around 3% to 7.5% in the last month or so.
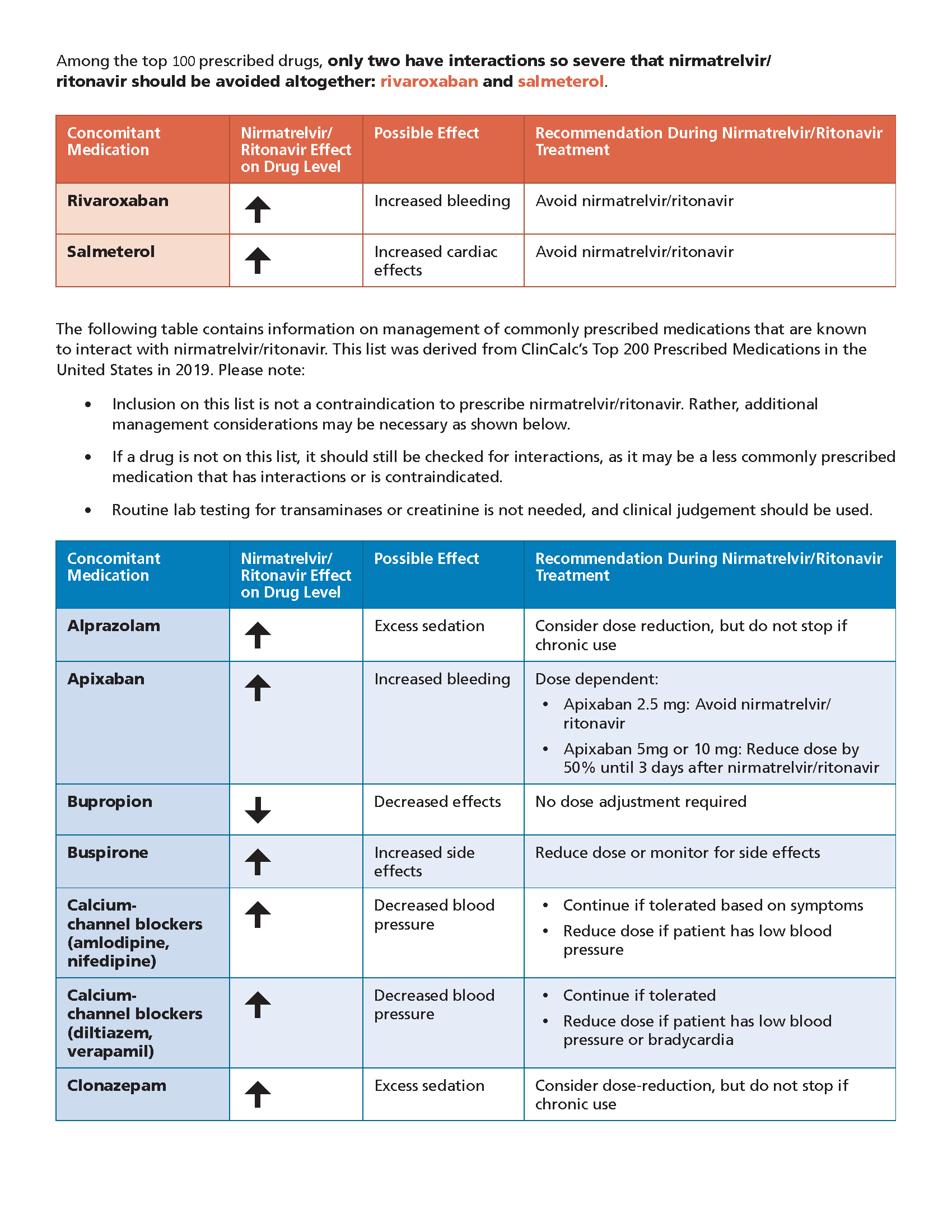
Paxlovid may be prescribed for an individual patient by physicians, advanced practice registered nurses, and physician assistants that are licensed or authorized under state.

Management of Drug Interactions With Nirmatrelvir/Ritonavir (Paxlovid, You can review the national clinical evidence taskforce. The documented share of covid tests in california that came back positive has risen from around 3% to 7.5% in the last month or so.
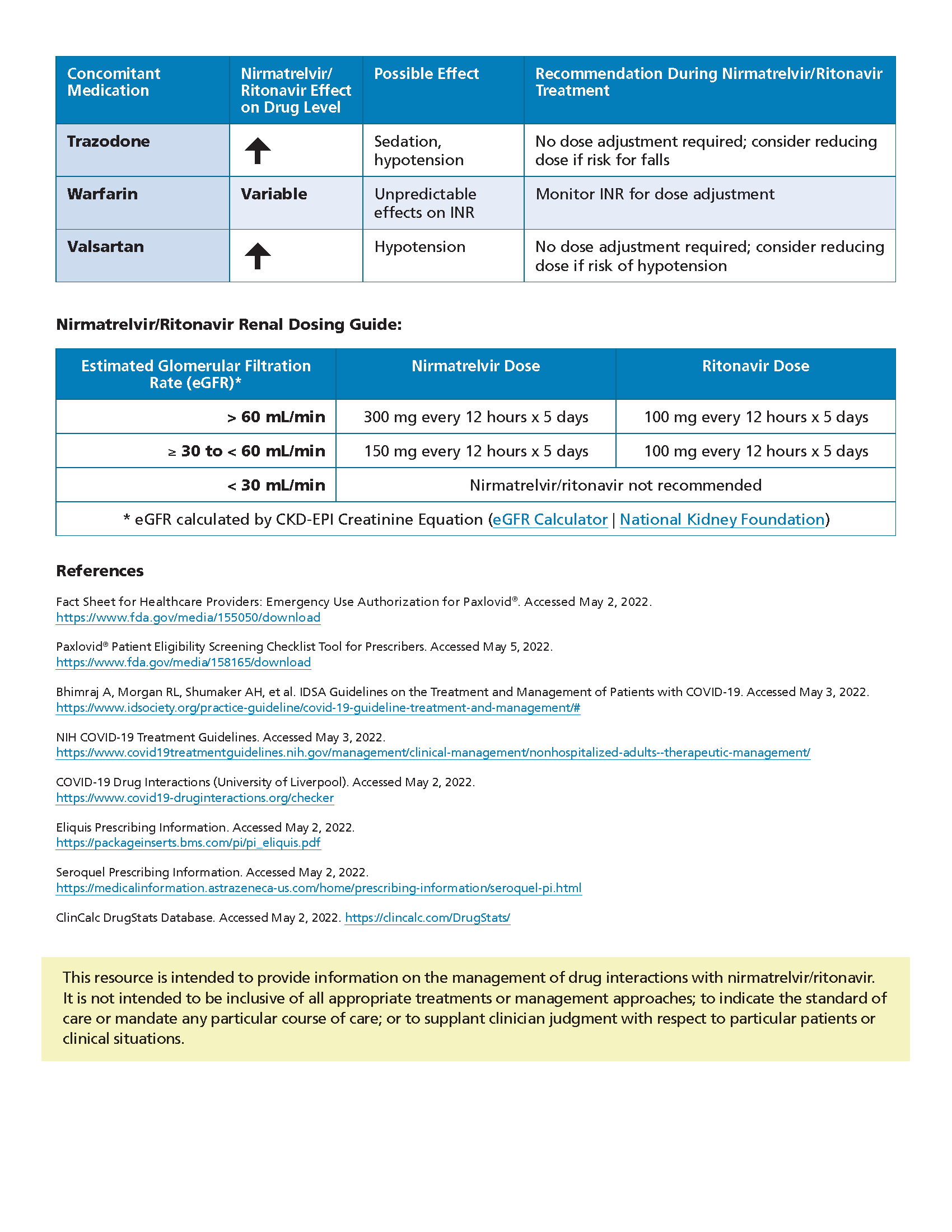
Paxlovid Healthify, Nice already recommends paxlovid (also called nirmatrelvir plus ritonavir and made by pfizer) for an estimated 3.9 million people who do not need supplemental. The recommendations are intended to provide guidance for prescribers on antiviral therapy (nirmatrelvir/ritonavir and remdesivir) for adults with mild to moderate.

Management of Drug Interactions With Nirmatrelvir/Ritonavir (Paxlovid, You can review the national clinical evidence taskforce. Treatment of flu with antiviral medications can lessen symptoms.

Paxlovid for a Patient on a DOAC Ontario COVID19 Science Advisory Table, The recommendations are intended to provide guidance for prescribers on antiviral therapy (nirmatrelvir/ritonavir and remdesivir) for adults with mild to moderate. One of the best tools for preventing severe complications from covid infection is the prescription antiviral drug paxlovid.

Paxlovid Skippack Pharmacy, [01/29/2025] in december 2025, fda authorized paxlovid for emergency use for the treatment of adults and pediatric patients (12 years of age and older weighing at least 40. There are new canadian recommendations for who should actually get paxlovid at this point, guided by a growing body of research suggesting the drug’s life.

Antiviral / Paxlovid Access Hamilton Family Medicine, The recommendations are intended to provide guidance for prescribers on antiviral therapy (nirmatrelvir/ritonavir and remdesivir) for adults with mild to moderate. When prescribing paxlovid via the pbs, ensure the patient meets the eligibility criteria outlined on the pbs listing.

Patient information for Paxlovid GOV.UK, The recommendations are intended to provide guidance for prescribers on antiviral therapy (nirmatrelvir/ritonavir and remdesivir) for adults with mild to moderate. [01/29/2025] in december 2025, fda authorized paxlovid for emergency use for the treatment of adults and pediatric patients (12 years of age and older weighing at least 40.

Antiviral treatment (Paxlovid) is available for higherrisk individuals, Paxlovid will remain free for people without health insurance through 2028, and beginning in 2025, it will be free for people who are underinsured, also through. The recommendations are intended to provide guidance for prescribers on antiviral therapy (nirmatrelvir/ritonavir and remdesivir) for adults with mild to moderate.

Paxlovid for a Patient on a DOAC Ontario COVID19 Science Advisory Table, When prescribing paxlovid via the pbs, ensure the patient meets the eligibility criteria outlined on the pbs listing. Treatment of flu with antiviral medications can lessen symptoms.
Rebound after taking Paxlovid for COVID19, The recommendations are intended to provide guidance for prescribers on antiviral therapy (nirmatrelvir/ritonavir and remdesivir) for adults with mild to moderate. You can review the national clinical evidence taskforce.
As of october 13, 2025, hhs and pfizer reached an agreement extending patient access to paxlovid, maximizing taxpayer investment, and beginning paxlovid’s transition to the.
Paxlovid will remain free for people without health insurance through 2028, and beginning in 2025, it will be free for people who are underinsured, also through.